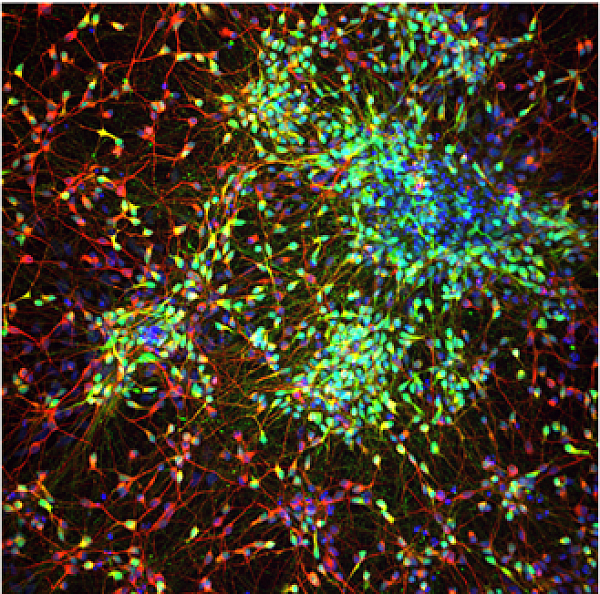
This study will investigate functional and genetic characteristics of the schizophrenia associated CNV of 16p11.2 in the development of patient derived striatal neuron networks in vitro. Schizophrenia is a chronic and serious mental disorder that profoundly affects an individual’s life. Although several antipsychotic medications have been developed, they only treat a subset of symptoms and can cause significant side-effects. Thus, more detailed studies are needed to determine the molecular deficits causing neuronal dysfunction in the patient brain and to find new molecular targets for development of more effective and disease modifying pharmacological interventions. Deficits in dopaminergic neuron function and abnormal dopamine signaling have been shown to be crucial in the development of schizophrenia. Medium spiny neurons mediate dopamine (DA) signaling in striatum via expression of dopaminergic receptors D1 and D2. At the transcriptional level, several genetic studies have shown that 16p11.2 duplication is one of the most common CNVs associated with schizophrenia. MAPK3 is located on the 16p11.2 gene region, and it encodes ERK1-MAP-kinase, which has been shown to regulate survival and function of the neurons in striatum. Previous mouse studies have also indicated that antipsychotic drugs regulate ERK-pathway activation in the striatal neurons. However, the detailed molecular mechanisms affecting development of striatal neuronal networks with 16p11.2 duplication have not been investigated in human cells. Here, we will investigate the role of 16p11.2 duplication in hiPSC-derived medium spiny neurons, and determine whether the synaptic development and function is altered in these cells leading to schizophrenia associated MAPK3-ERK activation.